An emulsion is a dispersion of two or more immiscible materials, where one phase, also known as the internal phase, is dispersed in the continuous or external phase
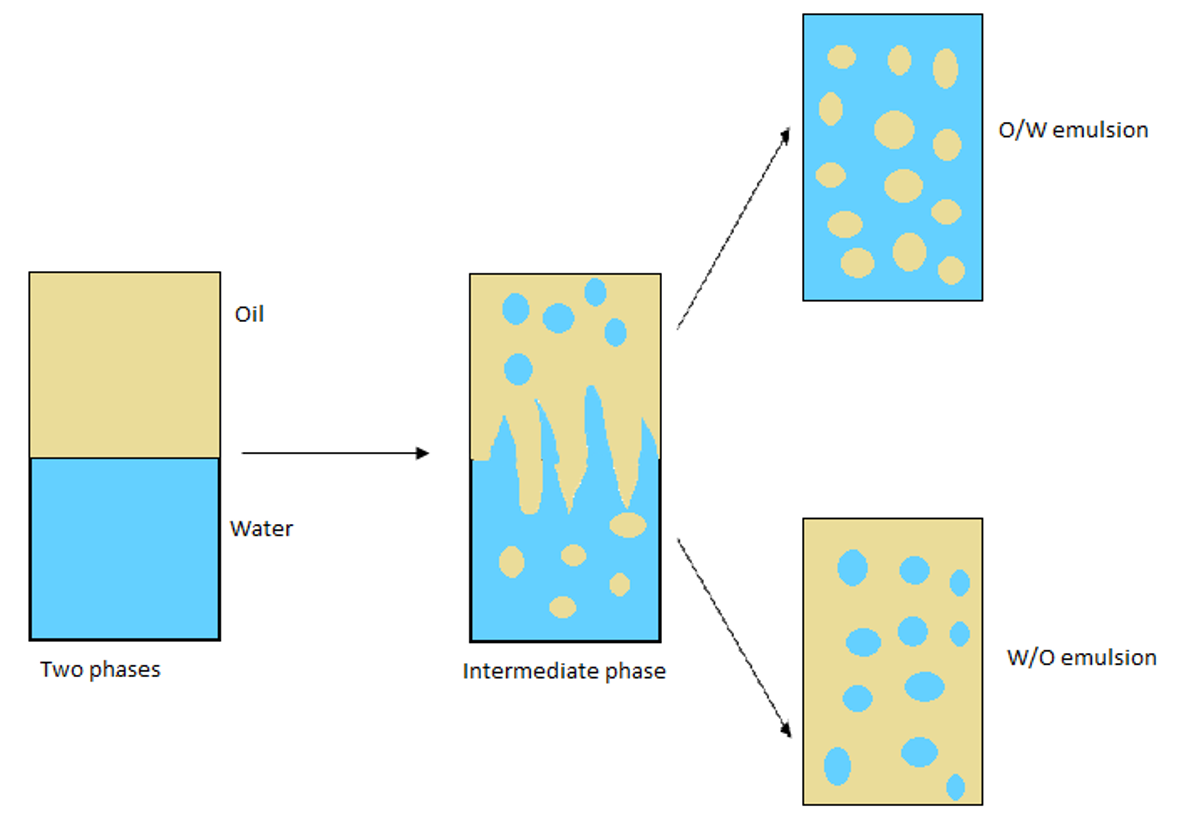
Cosmetic emulsions are classified as oil in water (O/W), water in oil (W/O) and water in silicone (W/Si). Multiple emulsions such as oil water in oil (W/O/W) are also possible. Oil in water emulsions are the most common due to preferable cost and light skin feel.
Emulsification consists of dispersing one fluid into another, no miscible ones, via creation of an interface.
Emulsification methods can be classified in 2 main categories:
a.- Mechanical methods: Energy to break droplet is mechanical
- Mechanical Mixing using different type of impeller
- High Pressure Homogenization
- Membrane Permeation Emulsification
b.- Physico-Chemical methods: energy is coming from modification of chemical and physical parameters.
- Phase Inversion Temperature
- Phase Inversion Composition
Emulsion is an out of equilibrium system: metastable
 The path to make the emulsion has as much impact on emulsion characteristics and properties as the emulsion composition.
The path to make the emulsion has as much impact on emulsion characteristics and properties as the emulsion composition.
Emollients are ingredients found in the oil phase and they provide skin protection and prevent skin dryness.
Key parameters for emollient selection:
- Sensorial:
Spreadability (rub-out), gloss (after-feel), oiliness (after-feel), firmness (pick-up), absorbency(rub-out),…
- Clinical:
Moisturising, substantivity, lubricity, softening,…
- Physical:
Compatibility, viscosity, spreading coefficient, polarity,…
General characteristics of a good emollient:
- Good chemical stability
- Good compability in the formulation
- Ease of incorporation
- Good spreadability (High spreading coefficient, Low interfacial tension, Low surface tension)
- Low toxicity (Non comedogenic)
- Biodegradable
- Global approval as PC ingredient
Esters are biocompatible ingredients that increase the static skin water level when applied and help to restore the natural hydrophobic barrier.
Functions as a cosmetic ingredient:
Biological or Health related functions:
- Lubricants in the stratum
- Restoration of the skin barrier function
- Preventing the penetration of exogenic undesired factors
- Regulation of the skin hydration
- Moisturization
- Skin adhesion= substantivity
- Elasticity
- Refattening
- Occlusivity= film forming=protection
- Skin penetration enhancer
Functional properties:
- Solvent/carrier for actives:
- Chemical sunscreens agents (SPF Boosting)
- Antiperspirant
- Water-repellency
- Dispersing medium:
- Pigments
- Physical sunscreen
- Gloss
Features:
- Spreadability
Spreading on the skin is influenced by:
Viscosity of the emollient
Surface and interfacial tension of the emollient
Water content of the Stratum Corneum
Amount of sebum on the skin
Cascading theory:
Cascading theory means select a number of emollients so that the sensorial feel is spread throughout the time of application.
Perfect combination of emollients
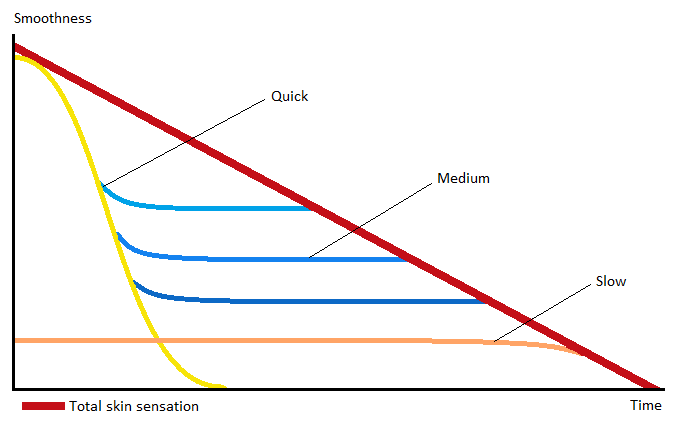
Combination of emollients depending on their spreadability: High, medium and low.
Polarity
Emollients according to their polarity
Applications:
Any type of emulsions (facial creams, body milks, sunscreens, make-up’s, make-up removers, baby products, anti-perspirants, hair mascara & conditioners,…)
Used level is dependent on formulation type: 1 to 30%.
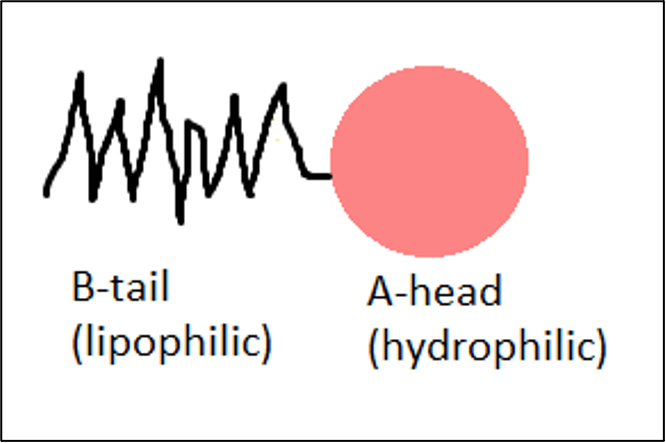
Emulsifiers are compounds able to stabilize the dispersed droplets in the continuous phase. Emulsifiers are molecules consisting of waterloving (hydrophilic) part and water-hating but oilloving (lipophilic) part. With their lipophilic part emulsifiers wrap around and incorporate oil drops thereby preventing them from reunite again to form a separate oily phase. In this way, the oil particles are shielded from each other resulting in a stable emulsion.
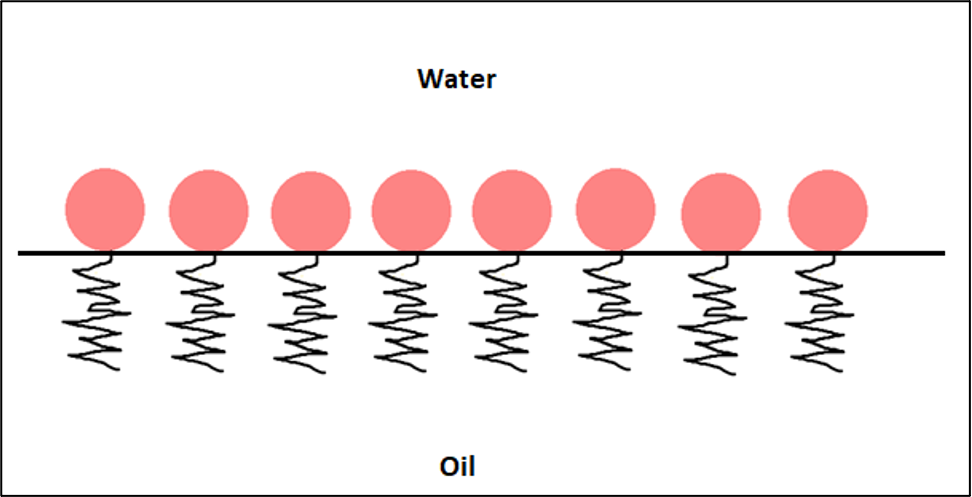
Characteristics of a suitable emulsifier:
- High interface activity
- High spreadability at the liquid/liquid interface
- Sufficient diffusion to the interface
- Sufficient solubility in the external phase
- High elasticity of the formed emulsifier film
The functions of surface-active agents to provide stability to dispersed droplets are as following:
- Reduction of the interfacial tension.
- Form coherent monolayer to prevent the coalescence of two droplet when they approach each other.
- Provide surface charge which cause repulsion between adjust particles.
Combination of surface-active agent is used most frequently. The combination should form film that closely packed and condensed.
Emulsifier’s classification:
O/W emulsifiers: High HLB value
W/O emulsifiers: Low HLB value
Emulsifier’s type:
- Anionic Emulsifiers (O/W):
Anionic O/W emulsifiers can be used to obtain very stable emulsions because they can build an electrical double layer around the droplets, which prevents the droplets from coalescing. They carry a net negative charge in solution and because of that they are sensitive to low pH and electrolytes.
The most important anionic O/W emulsifier is soap, which forms the stearate creams. Neutralized with potassium hydroxide, the stearate is alkaline. The use of triethanolamine as a neutralizing agent is more common. A disadvantage of stearic acid is the typical “soap-up” effect, a type of foaming that occurs when the cream is rubbed into the skin. To prevent this, silicon oil is frequently added to the formulation.
In principle, all anionic surfactants can be used. However, due to the fact that the soaps are relatively alkaline and fatty alcohol sulfates are irritant to the skin.
- Cationic Emulsifiers (O/W):
Cationic emulsifiers have generally been avoided in skin-care products because they are often more irritant compared with anionic emulsifiers. They are important in hair-care formulations, where they act as conditioners and anti-static agents. These are commonly called quaternium ammonium compounds.
Cationic emulsifiers function very well in formulations that are desired to stay on the skin for a long time such as sunscreens, long-wear make-up and barrier creams. This is because they are positive, cationic charge adheres these products strongly to the surface of the skin, resisting wash-off and wear.
A practical example of where cationic emulsifiers has proved very helpful, is in preventing sand from sticking to a freshly-sun-screened body. Thanks to its anti-static capacity.
It is important to note that, in using a cationic emulsifier system the formulator does not rule out the use of pretty much all grades of carbomer, and even anionic thickening agents such as xanthan and unmodified guar.
- Non-ionic Emulsifiers (O/W):
Non-ionics remain the first choice go-to emulsifiers for most applications due to their flexibility and low potential for chemical interaction. Non- ionic emulsifiers are those that are free from any external electrical charge caused by free ions. Examples of these are:
- Polysorbate 80
- Polysorbate 20
These are familiarly known as Tweens, a common trade name.
A wealth of non-ionic O/W emulsifiers is available. These are mainly PEG derivatives such as ethoxylates or PEG esters. Typical O/W emulsifiers are:
- ethoxylated fatty alcohol
- PEG esters of fatty acids
- Ethoxylated sorbitan esters
- Ethoxylated monoglycerides
- Ethoxylated castor-oil derivates
The degree of ethoxylation determines the primary properties of that part of the molecule that results in the HLB classification. Good W/O emulsifiers can be found in the HLB range between 8 and 18. Contrary to anionic emulsifiers, the non-ionics are unaffected b changes in pH and, in the case of pure ethers, they can also be used at extreme pH values.
The key benefit of a non-ionic emulsifier is its robust salt tolerance.
An emulsion is a combination of two immiscible phases held together by what to many looks and feels like magic.
The surface tension between oil and water is so high that they do not mix unless you add an emulsifier.
Glycerin, Propylene Glycol and Ethanol are common additives in a cosmetics product and can all impact on surface tension. All these ingredients decrease the polarity of the water phase, and the influence of that decrease changes depending on the quantity of additive present, although the relationship between dose and effect is not strictly linear.
Impact of Ions in Water phase:
We often talk about salt and saltiness in formulating, but what we really talk about much of the time is the ionic strength of continuous phase.
Ionic charge in the water phase can help increase intramolecular bonding and can also help in the formation of an electric double layer around the dispersed phase.
Adding too many ions into the continuous phase will give the product a sticky/tacky /salty feel when applied.
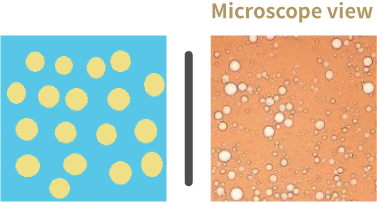
Oil in Water emulsion (O/W)
Oil in water emulsifiers create oil-in-water emulsions. In oil-in-water (O/W) emulsion systems, oil droplets are dispersed in water.
- Oils is the internal/dispersed phase whereas water is the external/continuous phase.
- O/W emulsifiers are more soluble in water than in oil.
- O/W emulsifiers have an HLB greater than 15.
| Advantages | Disadvantages |
|---|---|
| Good spreadability on the skin Good physical stability Stable | Vulnerable to microbial attack and bacterial contamination |
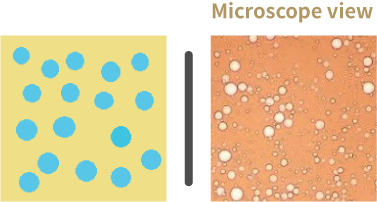
Water in Oil emulsion (W/O)
Water-in-oil emulsifiers help in producing water-in-oil (W/O) emulsions. In W/O emulsions, water droplets are dispersed in oil.
- Water is the internal/dispersed phase whereas oil is the external/continuous phase.
- W/O emulsifiers are more soluble in oil that water.
- They have HLB between 2.5-6 are non-ionic or polymeric.
| Advantages | Disadvantages |
|---|---|
| Water resistant Lustrous and glossy Smooth application Less susceptible to microbiological attack. | Heavy and greasy feeling on the skin. Difficult to obtain stable W/O systems, addition of stabilizers may be required. |
Oil in Water emulsion (O/W)
Water in Oil emulsion (O/W)
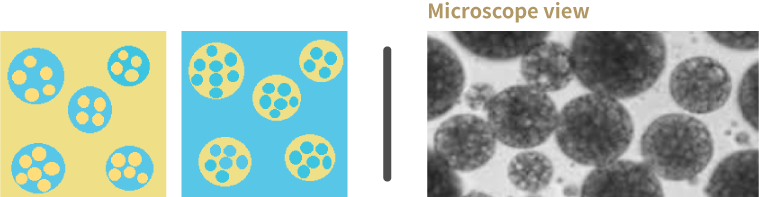
Multiple emulsion –double emulsion –W/O/W or O/W/O
Other type of emulsion
HIPE emulsion
High Internal Phase emulsion.
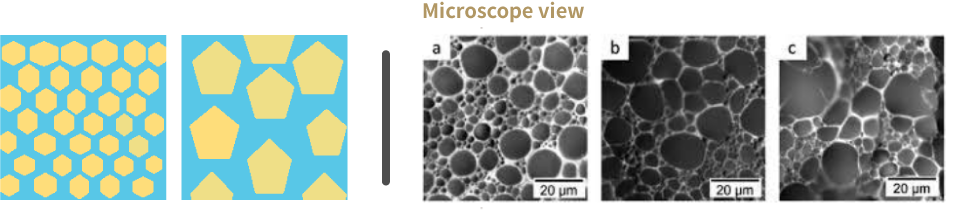
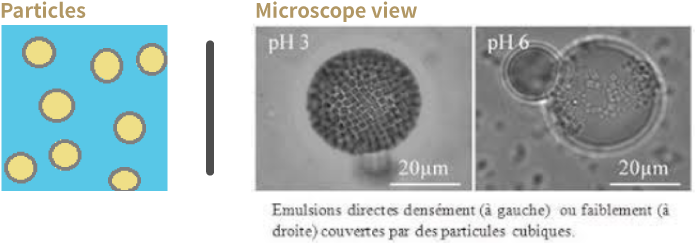
Pickering emulsion
Emulsion stabilized with particles rather than surfactant.
What is not an emulsion?

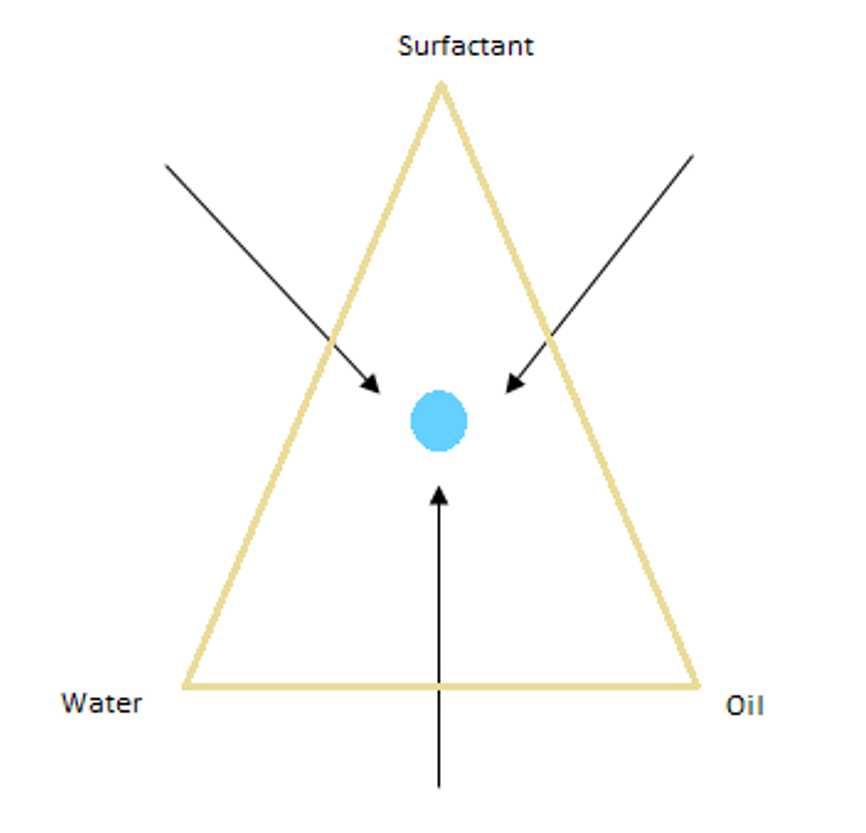
Micro-emulsion:
They are made of oil/water/surfactant but are at equilibrium. The structure is independent from path taken to make it and are STABLE.
* They usually contain a large amount of surfactant.
choice of emulsion type?
The choice of emulsion depends on:
- Properties and uses of final products
- The other material required to be present
W/O from O/W emulsion?
Dilution test with water:
- O/W can be diluted
- W/O cannot be diluted
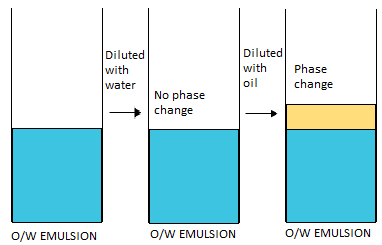
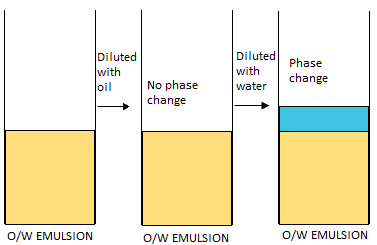
Conductivity test:
Water is good conductor of electricity whereas oil is non-conductor. Therefore, continuous phase of water runs electricity more than continuous phase of oil.
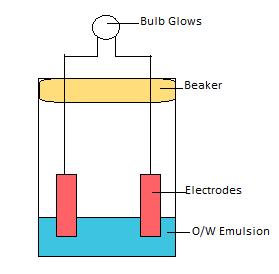
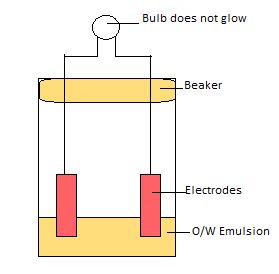
Visual observation for O/W emulsion (W/O emulsion undergo similar evolution).
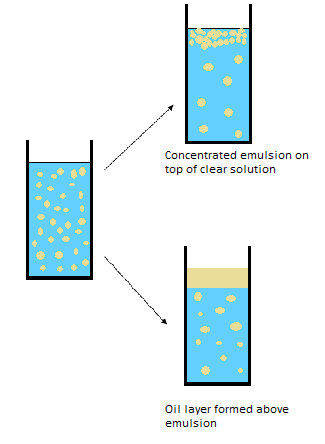
Mechanisms behind these observations are:
- Creaming / sedimentation
- Flocculation
- Coalescence
- Ostwald ripening
Destabilization Mechanism: Creaming / Sedimentation
Creaming is very common phenomena for emulsion, and it is due to the motion of emulsion droplets under effect of gravity.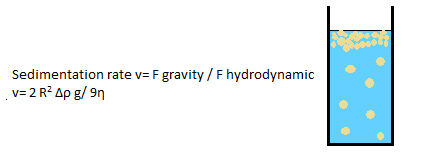
Can be avoided by:
- Increasing viscosity of continuous phase
- Continuous phase having a yield
- Changing density differences
- Reducing droplet size below 1mm
Destabilization Mechanism: Flocculation
Flocculation is due to the aggregation of droplets when they come to contact with each other.
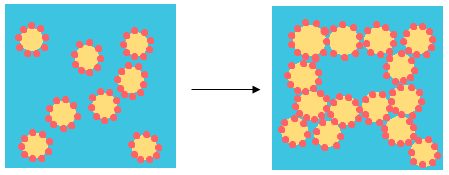
Flocculation results in modification of rheology, gasification or creaming and it may have different origins:
A. Van der Walls attraction when repulsion forces are lost:
- For emulsion stabilized by ionic surfactant, addition of salt reduces repulsion and may induce flocculation.
- For emulsion stabilized with non-ionic surfactant, interaction of surfactant with continuous phase may change by modification of parameters such as salt concentration, temperature, …
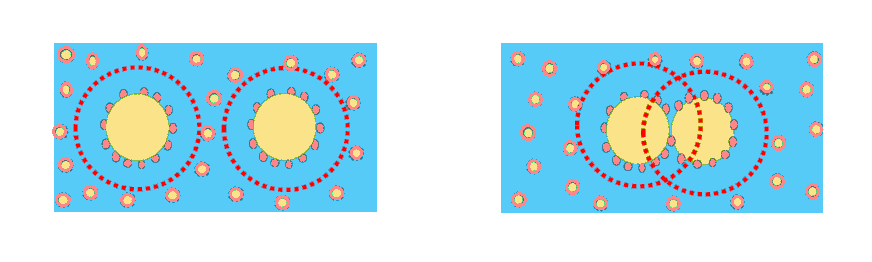
* This type of flocculation may be avoided by controlling Ionic Strength and compatibility of surfactant with additives present in continuous phase.
B. Bridging of droplets by polymer:
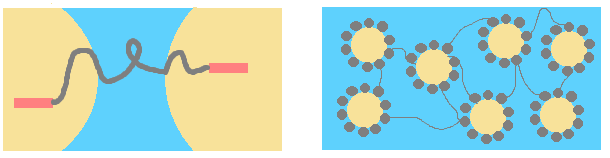
* To avoid it, nature of interaction between polymer and surfactant has to be modified.
C. Depletion:
Depletion induced flocculation occurs for mixture of small and large particles such as micelles and oil droplets.
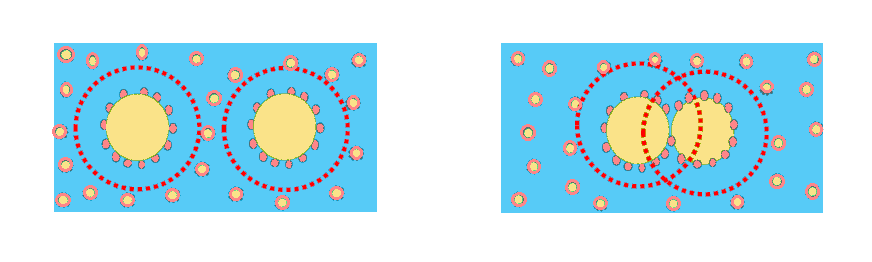
* This may be avoided by removing surfactant excess.
Destabilization Mechanism: Coalescence
Coalescence results from merge of droplets. This reduces the total surface resulting in an increase of droplets size. If the process continues an oil layer forms at the surface of the sample.
Coalescence process can be summarized in three steps:
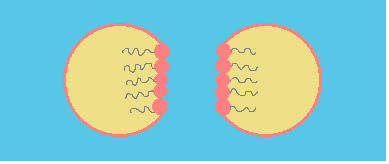
a.- Approach: droplets come to contact. This step is determined by droplet interaction.
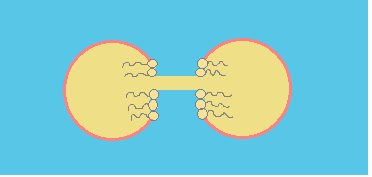
b.- Hole opening: an oil bridge is established between the two drops.
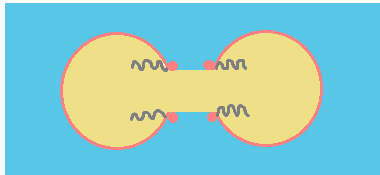
c.- Hole growth: the bridge grows and eventually the droplets completely merge.
d.- Hole opening: this step needs re location of surfactant which can happen by compression of interface layer or dispersion in internal phase or continuous phase.
e.- Hole growth: this is directly related to the preferred curvature. When preferred curvature is toward the oil, it is opposite to the hole curvature so it is unfavorable. When preferred curvature is toward water similar to the hole there is no limitation for hole’s growth. 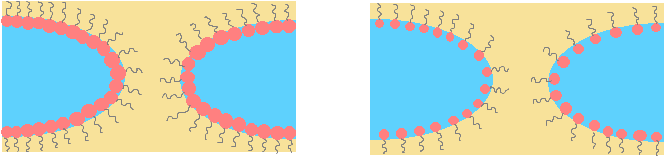
Can be avoided by:
- Surfactant concentration high enough to have a full surface coverage.
- Proper selection of surfactant to have a high spontaneous curvature: high HLB for O/W emulsion.
- Use of mixture of surfactant and co surfactant in order to increase film rigidity. This is commonly used in industry to prepare stable emulsion.
- Droplet size has also an impact on emulsion stability toward coalescence. Surface of contact between 2 large droplets is larger than between 2 small droplets leading to a higher probability for hole formation. In practice, emulsion above 10µm are difficult to stabilize.
Destabilization Mechanism: Ostwald ripening
Ostwald Ripening is a transfer of molecules from small droplets to large droplets through continuous phase. This phenomenon take place even at very low solubility of dispersed phase in continuous phase and leads to reduction of size of small droplets and growth of large droplets.
Driver for this phenomena is Laplace pressure which is inversely proportional to droplets diameter.
Ostwald ripening can be stopped by addition of salt or organic molecule having a different solubility in continuous phase: transfer will lead to difference of composition in droplets leading to a difference of osmotic pressure. Osmotic pressure will counterbalance Laplace pressure and will stop Ostwald ripening.
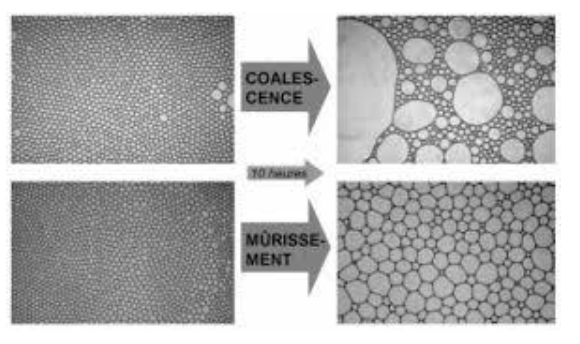
Ostwald ripening vs coalescence
Bancroft rule
The phase in which surfactant preferentially dissolves becomes the continuous phase during emulsification.
This rule results from an empirical observation but it is often verified.
Surfactant HLB
HLB is related to its solubility. A surfactant with low HLB will tend to be soluble in oil and one having high HLB will tend to be water soluble.
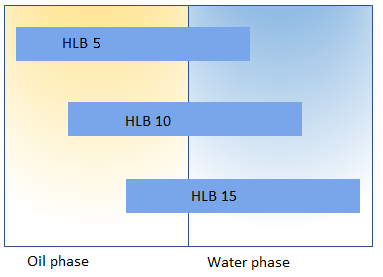
- HLB 3 to 8 are W/O emulsifiers
- HLB 8 to 16 are O/W emulsifiers
- HLB 13 to 16 are detergents
- HLB>15 are solubilizers
HLB of a mixture of surfactants is the weighted average of HLB of each surfactant of the blend.
The emulsion stability, generally, is achieved with a combination of two emulsifiers:
- Working with a pair of surfactants one with high HLB and other with low HLB.
- The HLB of the chosen emulsifiers has to be equal to the HLB of the oil emulfy.
- The solubility of the emulsifiers determines the type of continuous phase.
Required HLB for your System
R-HLB is determined experimentally for each System:
- Step 1: select surfactant having HLB from 8 to 16
- Step 2: prepare emulsion with a large excess of surfactant (10% vs oil) with different surfactant making HLB step of 2.
- - The most stable emulsion will define the R -HLB
- Step 3: prepare emulsion around R-HLB identified in step 2 in order to define more precisely R-HLB.
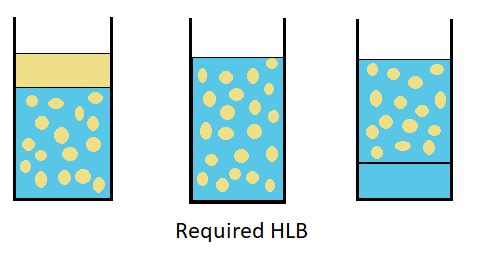
Emulsion for R-HLB identification can be made in a beaker by simple agitation or using the method that will be used for emulsion fabrication.
- For a given HLB, different surfactant may lead to different stability of emulsion. This is due to specific interaction of lipophilic part of surfactant with oil phases
- Surfactant optimization may be done after R-HLB determination by evaluation of several surfactants or combinations having HLB = R –HLB.
- Once the best surfactant selected, quantity of surfactant may be optimized.
Most common methods to characterize emulsions are:
Visual assessment:
Is key for emulsion characterization, creaming, sedimentation, flocculation and coalescence.
Optical microscopy:
It is used to observe droplet aspect and size, polydispersity and flocculation.
Particle size analysis using laser scattering or Coulter Counter:
Particle size and polydispersity, evolution of particle size over time, size distribution give information about coalescence and Ostwald ripening
Rheology measurements:
Flocculation and change of droplet size distribution lead to change in rheological properties of emulsion.
Storage stability at RT, 45°C and 4°C
- Most classical stability tests for any dispersion.
Stability under centrifugation
- Resistance of an emulsion to centrifugation gives information son the resistance of the interfacial film.
Freeze/Thaw cycles
- Freezing of internal phase, external phase or surfactant solidification may lead to emulsion breakage.


